Building Your Own Larry Hall Icyball
Building Your Own Larry Hall Icyball
DISCLAIMER: The following is information only on things I did. I make no claims expressed or implied as to the safety, usefulness, or accuracy of this information. (This is a high-risk activity. High pressures are involved. The Icyball can leak or explode resulting in death or serious injury. Exposure to ammonia can cause serious injury or death. We share these words and photos for information only. We make no claim as to their accuracy usefulness, or safety.)
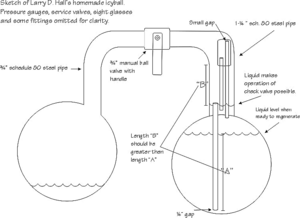
The cold ball is about 5" dia. and the hot ball is about 6" dia.. I used off the shelf steel pipe end caps (weld type), approx. 1/4" wall thickness if I remember right. The rest is just thick wall steel pipe and fittings. It's overbuilt for experimenting. On one charge it can keep an ice chest below 38 deg. F. for 24 hrs."
After my homemade Icyball was all assembled, I hydrostatically pressure tested it to 1,000 psi. This is done by completely filling the entire assembly with water, and then pressuring it up with some kind of hand pump. I had a pump I made. It works similar to a grease gun, but uses water. One of the pressure gauges I used is stainless; the other was made for ammonia systems. You can’t use copper or brass anywhere in the system. Some think Teflon sealer tape should not be used in a system like this but I found it to seal well and last for years. I tried another kind of thread compound, and it failed during the heat of regeneration.
I think the original Crosley Icyball charge was approximately 6 pounds anhydrous ammonia and 8 pounds water. My homemade Icyball was first evacuated, then charged with 1 pound 14 ounces of distilled water, then 1 pound 6 and 1/3 ounces ammonia or 57.2 parts by weight H20 and 42.8 parts by weight NH3. Or stated another way, the weight of the NH3 is 75% of the weight of the H20.
NH3, itself, is 61% the weight of H20 for the same volume so the actual volumes of water and ammonia in my homemade Icyball is .9 quarts water and 1.1 quarts anhydrous ammonia. It helps to know this in figuring what volumes of balls you want to weld up.
I have included two sketches of my homemade Icyball.
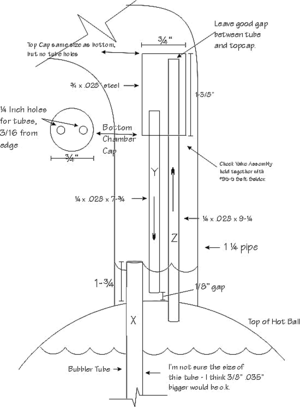
I have copies of two Icyball patents, one dated December 24, 1929, Number 1,740,737, and the other dated June 23, 1931, Number 1,811,523 (Improvements to the Icyball). I didn’t stick real close to the patent drawings. My check valve is similar to Number 17, 18 and 19 on the patent drawing. (See sketch Number 2). The function of Object 20 in the patent drawing, I believe, is to further separate water from the ammonia during regeneration. I don’t use this, and mine works okay without it. Some thought does need to be given to separating ammonia from the water during regeneration. You are actually distilling it. I experimented with putting wet rags around the tube above the hot ball during regeneration to help condense back the water and give a more pure ammonia solution in the cold ball. A person could easily write a whole book on all the details of what goes on in an Icyball.
The check valve: Mine is similar to the one on the patent drawing and is needed because during regeneration the ammonia vapors have to freely travel to the cold ball to condense. But during the cooling cycle, the ammonia vapors need to bubble up through the water to be better absorbed and to stir the water-ammonia mixture. During cooling, you can hear the ammonia slowly bubbling into the water. I built an Icyball without a check valve, and it would hardly work. A ball check valve would work in principle, but the problem is because of the open bubbler tube ("X" Sketch 2), the water-ammonia mixture would raise up this tube approximately 3 inches before creating enough back pressure to lift a quarter inch stainless steel ball off its seat (no spring) during regeneration. You could maybe make this work, but a bigger problem might be getting it to seal perfectly during cooling as even a very tiny seepage would defeat the bubbling feature. I thought of using a one quarter inch Teflon ball because of its lighter weight, but sealing is still a problem with so little weight or pressure on the ball. Perhaps an 0-ring seat might work, but in my research, the only rubber that would even come close to withstanding the heat and ammonia environment, is called "Aflas", but I didn't get around to trying this. The liquid check valve, however, works just fine. It seals perfectly and has no moving parts. It works as follows: (Sketch 2) During regeneration, the ammonia vapors go up tube "Z", down tube "Y", and up and over to the cold ball to condense. However, some ammonia and water condenses in the inch and a quarter tube (Sketch 1) and puddle up, one and three-fourths inches to the top of tube "X" (Sketch 2). During cooling, the ammonia vapors come back towards the hot ball and force some of the water in the aforementioned puddle to travel up tube "Y" far enough to create enough back pressure that the vapors will bubble up through tube "X". That is why length "B" should be greater than length "A" (Sketch 1). The reason for the 3/4 by 1-3/8" tube connecting the tops of tubes "Y" and "Z", I believe, is so a siphon won't easily develop between the two tubes. The lengths of my "Y" and "Z" tubes could be shorter if the bubbler tube was shorter, but I wanted the bubbler tube to go clear to the bottom. You'll notice in the patent drawing, the bubbler tube only goes part way down, and then a separate siphon tube helps to draw the solution from the bottom, I think. A good idea is to do what I did and make a prototype bubbler tube and check valve out of clear plastic tubing, then using plain water, blow and suck on it to see the action for yourself.
I have a three-quarter inch ball valve separating the two balls, but a needle valve might be better as I found that after regenerating, the ammonia would want to violently return to the other side and I would gradually open the valve.
I found that regenerating the system slowly is better than trying to regenerate it too fast (two hours is better than one hour).
I hope all this helps. Good luck!
Larry D. Hall
Tips from Other Builders
Texas Icyball
We followed Larry's design. We used steel brake lines for the small tubes inside the Icyball. We used high-silver-content solder for the delicate welds so that these welds would resist corrosion by the ammonia. We added a pressure relief valve, rated for Ammonia visible on the left side of the photo. It contains a safety valve designed to release at 300 psi. It is aimed down so that if it releases during the heating part of the cycle, it will discharge into the cooling water. We also added a small water dam in the horizontal pipe joining the two balls. It is like part 30 of the drawings in patent 1,811,523 of R Smith, June 23, 1931. It is in the left end of the horizontal tube, shaped like a crescent moon. It is silver-soldered into that tube. The dam helps to prevent water from traveling into the cold ball.
We fired the Icyball in two different setups: with a small Coleman dual fuel stove burning unleaded gasoline. We used a pail or a washtub filled with water for cooling and with a large propane burner and a trash can full of water. Both methods worked fine. The pail is such a small volume of water that it got hot and had to be dumped and refilled a few times. A low flame is the key to getting pure ammonia without much water in the cold ball. As long as ammonia is bubbling through during the heating phase (We listened at the elbow to the cold ball.) low heat will give the best results.
David Brydon
Thanks to David Brydon for having Larry Hall's sketches redrawn for posting.