Tablet
This article has multiple issues. Please help improve it or discuss these issues on the talk page. |
A tablet is a pharmaceutical dosage form. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted into a solid. The excipients can include diluents, binders or granulating agents, glidants (flow aids) and lubricants to ensure efficient tabletting; disintegrants to promote tablet break-up in the digestive tract; sweeteners or flavours to enhance taste; and pigments to make the tablets visually attractive. A polymer coating is often applied to make the tablet smoother and easier to swallow, to control the release rate of the active ingredient, to make it more resistant to the environment (extending its shelf life), or to enhance the tablet's appearance.
The compressed tablet is the most popular dosage form in use today. About two-thirds of all prescriptions are dispensed as solid dosage forms, and half of these are compressed tablets. A tablet can be formulated to deliver an accurate dosage to a specific site; it is usually taken orally, but can be administered sublingually, buccally, rectally or intravaginally. The tablet is just one of the many forms that an oral drug can take such as syrups, elixirs, suspensions, and emulsions. Medicinal tablets were originally made in the shape of a disk of whatever color their components determined, but are now made in many shapes and colors to help distinguish different medicines. Tablets are often stamped with symbols, letters, and numbers, which enable them to be identified. Sizes of tablets to be swallowed range from a few millimeters to about a centimeter. Some tablets are in the shape of capsules, and are called "caplets". Medicinal tablets and capsules are often called pills. This is technically incorrect, since tablets are made by compression, whereas pills are ancient solid dose forms prepared by rolling a soft mass into a round shape. Other products are manufactured in the form of tablets which are designed to dissolve or disintegrate; e.g. cleaning and deodorizing products.
Contents
[hide]Tabletting formulations
Some APIs may be tableted as pure substances, but this is rarely the case; most formulations include excipients. Normally, an pharmacologically inactive ingredient (excipient) termed a binder is added to help hold the tablet together and give it strength. A wide variety of binders may be used, some common ones including lactose, dibasic calcium phosphate, sucrose, corn (maize) starch, microcrystalline cellulose and modified cellulose (for example hydroxypropyl methylcellulose).
Often, an ingredient is also needed to act as a disintegrant to aid tablet dispersion once swallowed, releasing the API for absorption. Some binders, such as starch and cellulose, are also excellent disintegrants.
Small amounts of lubricants are usually added, as well. The most common of these is magnesium stearate; however, other commonly used tablet lubricants include stearic acid (stearin), hydrogenated oil, and sodium stearyl fumarate. These help the tablets, once pressed, to be more easily ejected from the die.
Advantages and disadvantages
Tablets are simple and convenient to use. They provide an accurately measured dosage of the active ingredient in a convenient portable package, and can be designed to protect unstable medications or disguise unpalatable ingredients. Colored coatings, embossed markings and printing can be used to aid tablet recognition. Manufacturing processes and techniques can provide tablets special properties, for example, sustained release or fast dissolving formulations.
Some drugs may be unsuitable for administration by the oral route. For example, protein drugs such as insulin may be denatured by stomach acids. Such drugs cannot be made into tablets. Some drugs may be deactivated by the liver when they are carried there from the gastrointestinal tract by the hepatic portal vein (the "first pass effect"), making them unsuitable for oral use. Drugs which can be taken sublingually are absorbed through the oral mucosae, so that they bypass the liver and are less susceptible to the first pass effect. The oral bioavailability of some drugs may be low due to poor absorption from the gastrointestinal tract. Such drugs may need to be given in very high doses or by injection. For drugs that need to have rapid onset, or that have severe side effects, the oral route may not be suitable. For example salbutamol, used to treat problems in the pulmonary system, can have effects on the heart and circulation if taken orally; these effects are greatly reduced by inhaling smaller doses direct to the required site of action.
Tablet properties
Tablets can be made in virtually any shape, although requirements of patients and tableting machines mean that most are round, oval or capsule shaped. More unsusual shapes have been manufactured but patients find these harder to swallow, and they are more vulnerable to chipping or manufacturing problems.
Tablet diameter and shape are determined by the machine tooling used to produce them - a die plus an upper and a lower punch are required. This is called a station of tooling. The thickness is determined by the amount of tablet material and the position of the punches in relation to each other during compression. Once this is done, we can measure the corresponding pressure applied during compression. The shorter the distance between the punches, thickness, the greater the pressure applied during compression, and sometimes the harder the tablet. Tablets need to be hard enough that they don't break up in the bottle, yet friable enough that they disintegrate in the gastric tract.
Tablets need to be strong enough to resist the stresses of packaging, shipping and handling by the pharmacist and patient. The mechanical strength of tablets is assessed using a combination of (i) simple failure and erosion tests, and (ii) more sophisticated engineering tests. The simpler tests are often used for quality control purposes, whereas the more complex tests are used during the design of the formulation and manufacturing process in the research and development phase. Standards for tablet properties are published in the various international pharmacopeias (USP/NF, EP, JP, etc).
Lubricants prevent ingredients from clumping together and from sticking to the tablet punches or capsule filling machine. Lubricants also ensure that tablet formation and ejection can occur with low friction between the solid and die wall.
Common minerals like talc or silica, and fats, e.g. vegetable stearin, magnesium stearate or stearic acid are the most frequently used lubricants in tablets or hard gelatin capsules.[citation needed]
Manufacturing
Manufacture of the tableting blend
In the tablet pressing process, the main guideline is to ensure that the appropriate amount of active ingredient is in each tablet. Hence, all the ingredients should be well-mixed. If a sufficiently homogenous mix of the components cannot be obtained with simple blending processes, the ingredients must be granulated prior to compression to assure an even distribution of the active compound in the final tablet. Two basic techniques are used to granulate powders for compression into a tablet: wet granulation and dry granulation. Powders that can be mixed well do not require granulation and can be compressed into tablets through direct compression.
Wet granulation
Wet granulation is a process of using a liquid binder to lightly agglomerate the powder mixture. The amount of liquid has to be properly controlled, as over-wetting will cause the granules to be too hard and under-wetting will cause them to be too soft and friable. Aqueous solutions have the advantage of being safer to deal with than solvent-based systems but may not be suitable for drugs which are degraded by hydrolysis.
- Procedure
- Step 1: The active ingredient and excipients are weighed and mixed.
- Step 2: The wet granulate is prepared by adding the liquid binder–adhesive to the powder blend and mixing thoroughly. Examples of binders/adhesives include aqueous preparations of cornstarch, natural gums such as acacia, cellulose derivatives such as methyl cellulose, gelatin, and povidone.
- Step 3: Screening the damp mass through a mesh to form pellets or granules.
- Step 4: Drying the granulation. A conventional tray-dryer or fluid-bed dryer are most commonly used.
- Step 5: After the granules are dried, they are passed through a screen of smaller size than the one used for the wet mass to create granules of uniform size.
Low shear wet granulation processes use very simple mixing equipment, and can take a considerable time to achieve a uniformly mixed state. High shear wet granulation processes use equipment that mixes the powder and liquid at a very fast rate, and thus speeds up the manufacturing process. Fluid bed granulation is a multiple-step wet granulation process performed in the same vessel to pre-heat, granulate, and dry the powders. It is used because it allows close control of the granulation process.
Dry granulation
Dry granulation processes create granules by light compaction of the powder blend under low pressures. The compacts so-formed are broken up gently to produce granules (agglomerates). This process is often used when the product to be granulated is sensitive to moisture and heat. Dry granulation can be conducted on a tablet press using slugging tooling or on a roll press called a roller compactor. Dry granulation equipment offers a wide range of pressures to attain proper densification and granule formation. Dry granulation is simpler than wet granulation, therefore the cost is reduced. However, dry granulation often produces a higher percentage of fine granules, which can compromise the quality or create yield problems for the tablet. Dry granulation requires drugs or excipients with cohesive properties, and a 'dry binder' may need to be added to the formulation to facilitate the formation of granules.
Granule lubrication
After granulation, a final lubrication step is used to ensure that the tableting blend does not stick to the equipment during the tableting process. This usually involves low shear blending of the granules with a powdered lubricant, such as magnesium stearate or stearic acid.
Manufacture of the tablets
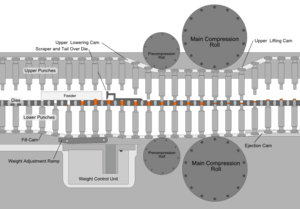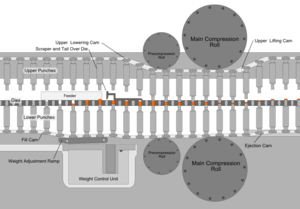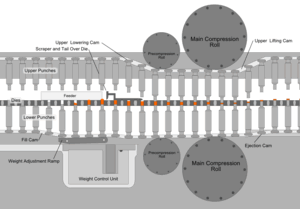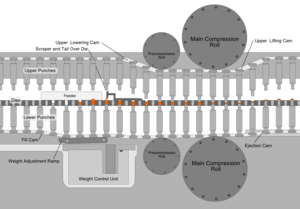
Whatever process is used to make the tableting blend, the process of making a tablet by powder compaction is very similar. First, the powder is filled into the die from above. The mass of powder is determined by the position of the lower punch in the die, the cross-sectional area of the die, and the powder density. At this stage, adjustments to the tablet weight are normally made by repositioning the lower punch. After die filling, the upper punch is lowered into the die and the powder is uniaxially compressed to a porosity of between 5 and 20%. The compression can take place in one or two stages (main compression, and, sometimes, pre-compression or tamping) and for commercial production occurs very fast (500–50 msec per tablet). Finally, the upper punch is pulled up and out of the die (decompression), and the tablet is ejected from the die by lifting the lower punch until its upper surface is flush with the top face of the die. This process is simply repeated many times to manufacture multiple tablets.
Common problems encountered during tablet manufacturing operations include:
- poor (low) weight uniformity, usually caused by uneven powder flow into the die
- poor (low) content uniformity, caused by uneven distribution of the API in the tableting blend
- sticking of the powder blend to the tablet tooling, due to inadequate lubrication, worn or dirty tooling, and sub-optimal material properties
- capping, lamination or chipping. Such mechanical failure is due to improper formulation design or faulty equipment operation.
Tablet compaction simulator
Tablet formulations are designed and tested using a laboratory machine called a Tablet Compaction Simulator or Powder Compaction Simulator. This is a computer controlled device that can measure the punch positions, punch pressures, friction forces, die wall pressures, and sometimes the tablet internal temperature during the compaction event. Numerous experiments with small quantities of different mixtures can be performed to optimise a formulation. Mathematically corrected punch motions can be programmed to simulate any type and model of production tablet press. Initial quantities of active pharmaceutical ingredients are very expensive to produce, and using a Compaction Simulator reduces the amount of powder required for product development.
Tablet presses
Tablet presses, also called tableting machines, range from small, inexpensive bench-top models that make one tablet at a time (single-station presses), with only around a half-ton pressure, to large, computerized, industrial models (multi-station rotary presses) that can make hundreds of thousands to millions of tablets an hour with much greater pressure. The tablet press is an essential piece of machinery for any pharmaceutical and nutraceutical manufacturer. Common manufacturers of tablet presses include Fette, Korsch, Kikusui, Manesty and Courtoy. Tablet presses must allow the operator to adjust the position of the lower and upper punches accurately, so that the tablet weight, thickness and density can each be controlled. This is achieved using a series of cams, rollers, and/or tracks that act on the tablet tooling (punches). Mechanical systems are also incorporated for die filling, and for ejecting and removing the tablets from the press after compression. Pharmaceutical tablet presses are required to be easy to clean and quick to reconfigure with different tooling, because they are usually used to manufacture many different products.
Tablet coating
Many tablets today are coated after being pressed. Although sugar-coating was popular in the past, the process has many drawbacks. Modern tablet coatings are polymer and polysaccharide based, with plasticizers and pigments included. Tablet coatings must be stable and strong enough to survive the handling of the tablet, must not make tablets stick together during the coating process, and must follow the fine contours of embossed characters or logos on tablets. Coatings are necessary for tablets that have an unpleasant taste, and a smoother finish makes large tablets easier to swallow. Tablet coatings are also useful to extend the shelf-life of components that are sensitive to moisture or oxidation. Opaque materials like titanium dioxide can protect light-sensitive actives from photodegradation[citation needed]. Special coatings (for example with pearlescent effects) can enhance brand recognition.
If the active ingredient of a tablet is sensitive to acid, or is irritant to the stomach lining, an enteric coating can be used, which is resistant to stomach acid, and dissolves in the less acidic area of the intestines. Enteric coatings are also used for medicines that can be negatively affected by taking a long time to reach the small intestine, where they are absorbed. Coatings are often chosen to control the rate of dissolution of the drug in the gastrointestinal tract. Some drugs will be absorbed better at different points in the digestive system. If the highest percentage of absorption of a drug takes place in the stomach, a coating that dissolves quickly and easily in acid will be selected. If the rate of absorption is best in the large intestine or colon, then a coating that is acid resistant and dissolves slowly would be used to ensure it reached that point before dispersing. The area of the gastrointestinal tract with the best absorption for any particular drug is usually determined by clinical trials.
Pill-splitters
It is sometimes necessary to split tablets into halves or quarters. Tablets are easier to break accurately if scored, but there are devices called pill-splitters which cut unscored and scored tablets. Tablets with special coatings (for example enteric coatings or controlled-release coatings) should not be broken before use, as this will expose the tablet core to the digestive juices, short-circuiting the intended delayed-release effect.
See also
References
- Kibbe, A.H., ed. Handbook of Pharmaceutical Excipients. 3rd Edition ed. 2000, American Pharmaceutical Association & Pharmaceutical Press: Washington, DC & London, UK.
- Hiestand, E.N., 2003. Mechanics and physical principles for powders and compacts, SSCI Inc., West Lafayette, In, USA.
- United States Pharmacopeia, United States Pharmacopeia / National Formulary (USP25/NF20). 2002, Rockville, MD: United States Pharmacopeia Convention Inc.
da:Tablet de:Tablette et:Tablett es:Comprimido eo:Pilolo fr:Comprimé ko:알약 id:Tablet it:Compressa he:גלולה lt:Tabletė hu:Tabletta nl:Tablet ja:錠剤 no:Tabletter nn:Tablett pl:Tabletka pt:Comprimido ru:Таблетки sl:Tableta fi:Tabletti sv:Tablett zh:片剂
- Pages with broken file links
- Articles needing additional references from July 2006
- All articles needing additional references
- Articles needing cleanup from October 2009
- All articles needing cleanup
- All articles with unsourced statements
- Articles with unsourced statements from June 2010
- Articles with invalid date parameter in template
- Articles with unsourced statements from September 2009
- Chemical engineering
- Pharmaceutical industry
- Pharmacology
- Drug delivery devices
- Dosage forms
- 2Fix